This section is for information purpose only. For actual treatment, please consult with your doctor.
Treatment of Palmoplantar Pustulosis
The pathogenesis of palmoplantar pustulosis (PPP) has not been fully elucidated, which has resulted in a delay in the standardization of its treatment. Based on the clinical data accumulated over its long history, development of the first treatment guidelines has started. A clinical trial of a new therapeutic agent was conducted with the cooperation of patients based on recently obtained scientific data, and a new treatment, i.e., a biologics targeting one of the causative agents of PPP, was introduced in 2018 1,2). Furthermore, safe and effective therapeutic agents are expected to be developed in the future. Development of the treatment guidelines for PPP is still ongoing.
The treatment of PPP is substantially different from that of rheumatoid arthritis or psoriasis, which are representative conditions for targeted therapy with Biologics. PPP differs from psoriasis in that it is a diverse disease with a variety of trigger factors and that it is curable. Therefore, first, treatment options aimed at cure should be considered. The term “cure” here indicates not only resolution of skin symptoms, but also remission or prevention of pustulotic arthro-osteitis (PAO), which is reported to occur in 10% to 30% of patients.
The most important trigger factors for PPP are smoking 3,4) and focal infection 5-14). Focal infection refers to a lesion at some site in the body that is characterized by episodic inflammation or a totally asymptomatic infectious lesion, that can precipitate other diseases in distant organs. Japanese type of PPP is a representative disease triggered by focal infection, and in most cases, the focal infection is in the nose, mouth or throat, e.g., tonsillitis, periodontitis and sinusitis. If a focal infection triggering PPP is left untreated, bone and joint symptoms of PPP may appear or progress, or the number of sites of arthro-osteitis may increase, even if skin symptoms resolve. Since it appears that the bone and joint inflammation of PPP cannot be easily cured, unlike the skin inflammation, it is necessary to explore treatments to cure such inflammation before it becomes chronic. Furthermore, the presence/absence/nature of trigger factors for the development/exacerbation of PPP must be investigated in each patient presenting with the characteristic symptoms. Excessive immune responses to the factor may cause exacerbation of the skin symptoms and development/exacerbation of the osteoarticular symptoms of PPP, whereas elimination of the factor may be expected to result in cure. In patients without a trigger factor(s), the intrinsic immunological background may be significantly involved in the development of PPP, as in the case of psoriasis, and the treatment in such patients should be mainly aimed at suppressing the excessive immune responses.

It is speculated that in patients with longstanding trigger factors such as smoking, tonsillitis and periodontitis, events that change the immune status, such as mental stress, overwork, invasive dental treatment and acute tonsillitis can precipitate the development of PPP. Disturbed intestinal immunity, such as repeated diarrhea/constipation, is also known to trigger the development/exacerbation of PPP. In some cases, treatment of diabetes mellitus or autoimmune thyroiditis may lead to improvement of the symptoms. Smoking cessation or treatment of focal infection is of utmost importance for controlling the excessive immune responses. In addition, drug therapy, including local or systemic therapy, may be used, according to the severity, to suppress the excessive immune responses and control comorbidities and stress. As such, various factors trigger the onset/exacerbation of PPP. The patient must let the physician know about all possible aspects of the disease that he/she is suffering from, so as to enable him/her to select the optimal treatment. Investigation for the presence/absence/nature of possible trigger factors, including focal dental infection, blood tests to determine the presence/absence of comorbidities, and good patient-physician/nurse communication to confirm the appropriateness of the method of ointment application are of critical importance. The first step of treatment is the development of the appropriate therapeutic strategy by cooperation among the patient, doctor and nurse.
1) Terui T, et al. Efficacy and Safety of Guselkumab, an Anti–interleukin 23 Monoclonal Antibody, for Palmoplantar Pustulosis. A Randomized Clinical Trial. JAMA Dermatology 2018; 154 (3): 309-316
2) Terui T, et al. Efficacy and Safety of Guselkumab in Japanese patients with Palmoplantar Pustulosis. A Randomized Clinical Trial. JAMA Dermatol 2019; July 3. doi:10.1001/jamadermatol.2019.1394
3) Akiyama T, et al. The relationships of onset and exacerbation of pustulosis palmaris et plantaris to smoking and focal infections. J Dermatol. 1995; 22: 930-934
4) Hagforsen E, Michaelsson K, Lundgren E, et al: Women with palmoplantar pustulosis have distributed calcium homeostasis and a high prevalence of diabetes mellitus and psychiatric disorders: a case-control study. Acta derm Venereol 2005; 85: 225-232
5) Ono T. Evaluation of tonsillectomy as a treatment for pustulosis palmaris et plantaris. J Dermatol. 1977;4:163-72
6) Tsubota H, et al. Efficacy of tonsillectomy for improving skin lesions of pustulosis palmaris et plantaris -Evaluation of 289 cases at the Department of Otolaryngology of Sapporo Medical University-. J Otolaryngol Jpn 1994; 97: 1621-1630
7) Yamakita T, et al. Clinical effect of tonsillectomy in patients with pustulosis palmaris et plantaris (PPP). Jpn J Dermatol 2004; 114(14): 2319-2326
8) Izaki S, How to consider indication for tonsillectomy in palmoplantar pustulosis? ―from a dermatological perspective―. JOHNS 2008; 24(10): 1562-1565
9) Yamamoto Y, et al. Effects of treatment for focal dental infection on pustulosis palmaris et plantaris. Jpn J Dermatol. 2001;111: 821 -6
10) Ishiguro H, et al. Clinical study on the relationship between focal dental infection and palmoplantar pustulosis. Odontology 2000; 88(1): 256-271
11) Sakiyama H, et al. Possible involvement of T cell co-stimulation in pustulosis palmaris et plamtaris via the induction of inducible costimulatory in chronic focal infections. J Dermatol Sci 2008;50:197-207
12) Kikuchi N and Yamamoto T: Dental infecton as a triggering factor in palmoplantar pustulosis: Acta Derm Venereol 2013; 91: 721-722
13) Hamamoto M, et al. Outcome of tonsillectomy for palmoplantar pustulosis. Pract Otol (Kyoto) 1999; 92(2): 115-118
14) Fujihara K, etc. Palmoplantar pustulosis ―Focusing on treatment outcomes in tonsillectomy and non-tonsillectomy groups―. Pract Otol (Kyoto) 1999; 92(2): 119-122
➢ ●Treatment of palmoplantar pustulosis
➢ ●Treatment to eliminate trigger factors for the development/exacerbation of palmoplantar pustulosis
➢ ●Detection and treatment of asymptomatic focal infection
➢ ●Symptomatic drug therapy, etc.
➢ 1)Topical therapy (ointment)
➢ 2)Ultraviolet (UV) light therapy
➢ 3)Oral therapy
➢ 4)Biologics
➢ 5)Granulocytapheresis
➢ ●Advice in your daily life
➢Treatment of Palmoplantar Pustulosis
Treatment of palmoplantar pustulosis (PPP) includes removal of trigger factors precipitating the development/exacerbation of symptoms, often in combination with symptomatic therapy directed at alleviating the symptoms. Symptomatic therapy includes treatment for the skin symptoms, treatment for arthro-osteitis, and treatment directed against both the skin symptoms and the arthro-osteitis. Furthermore, precautions in daily life are also known to enhance the therapeutic efficacy of these treatments.

<Treatment algorithm>
*1 Koebner phenomenon: Refers to a condition in patients with some skin diseases in
which repeated stimulation of apparently normal skin induces the development of lesions.
➢ Treatment to eliminate trigger factors for the development/exacerbation of palmoplantar pustulosis (PPP)
This is the most important aspect of the treatment of Japanese type of PPP. It is aimed at curing PPP. PPP most commonly occurs in females in their 30s to 50s who smoke (> What causes PPP?). Therefore, the patients should first be counseled on smoking cessation. Patients with psychiatric disorders should consult their psychiatrist about the appropriateness/timing of smoking cessation.
In addition, in more than 75% of Japanese patients with PPP (> What causes PPP?), focal infection triggers the development of the lesions; therefore, efforts must be made by the physician to identify the infectious focus and then eliminate it.
In the treatment of focal infection, there are two important points, hardly recognized by even medical specialists, that must be borne in mind. The first is that the focal infection in patients with PPP is asymptomatic. It is difficult to detect these lesions due to the absence of inflammatory signs, such as swelling and pain; therefore, these lesions are often overlooked and left untreated. The lesions do not meet the current definition of inflammation. However, the presence of immunologically detectable inflammation has long been pointed out 11,15), and accumulating data suggest that treatment of these lesions also leads to resolution of other types of lesions in distant organs, such as PPP 5-12) and pustulotic arthro-osteitis (PAO)8,11,12) . However, since the mechanism of focal infections remains unclear, otolaryngologists and dentists remain skeptical that treatment of this condition may be excessive. However, the presence of multiple pustules on the hands and feet/pain of severe arthro-osteitis can greatly impair the quality of daily life of patients with PPP, robbing them of their jobs and a normal life. Since no tests can measure the intensity of pain, patients have to let their doctors or dentists know clearly how severe their pain is. In addition, once PPP progresses to bone and joint inflammation, such as spondylitis, the pathology cannot be reversed, so that it is important to determine the appropriate therapeutic strategy early in the course of the disease.
The second important point in the treatment of focal infection is to fully understand the predicted course of the symptoms after treatment; i.e., the skin and joint symptoms gradually resolve in approximately 1 to 2 year after the treatment of focal infection. Often, when the symptoms do not resolve by 1 to 2 weeks after the treatment of focal infection, many patients assume falsely that the treatment of the focal infection was ineffective. Inflammation of focal infections in cases of PPP is not associated with usual infection. Although bacteria are involved in the inflammation, the inflammation itself is considered to represent excessive immune responses to possibly commensal bacteria at the site of foci. While these bacteria are usually supposed to coexist peacefully with their host (immune tolerance*2), abnormal inflammatory response to these bacteria could be triggered by mistake (under conditions predisposing to breakdown of immune tolerance). It is difficult to completely eliminate commensal bacteria with antibiotics, therefore, the therapeutic strategy of removing the focal infection needs to be adopted. The goal of the treatment of focal infection is to remove the bacteria that trigger the inflammation (tonsillectomy, tooth extraction and apicoectomy) or replace these bacteria by normal commensal bacteria (dental root canal treatment, treatment of periodontitis, treatment of sinusitis, etc.) to eliminate new inflammatory signals. Spontaneous resolution of abnormal immune responses takes 1 to 2 years 16,17)after completion of the treatment for focal infection, and even if bacteriostatic antibiotics that reduce the amount of bacteria and have anti-inflammatory effects have been used concomitantly for 2 to 3 months, it often takes at least 6 months to approximately 1 year after the treatment for symptom resolution. However, during this period, the pattern of appearance of the pustules clearly begins to change. While the pustules continue to develop, the frequency of their appearance changes, with the interval between crops of pustules increasing progressively from every 1 to 2 weeks, to every 2 to 3 weeks, and then every 4 weeks. In addition, the number of new pustules in each crop also decreases. If these changes are noted, the symptoms could be expected to resolve altogether with time. One must wait patiently for complete resolution, while continuing to keep the skin moisturized, without scratching, scrubbing or rubbing(ointment).
If the symptoms worsen slightly a few days to 1 week after the start of treatment for focal infection, it rather represents a sign of improvement. It is called id reaction, and indicates that the lesion is associated with PPP and/or PAO. If the id reaction is severe, on the other hand, it may cause disability in daily life or progression of the osteoarticular symptoms, and patients should consult their doctors for treatment to suppress the inflammation.
If the pattern of pustule development remains unchanged for more than half a year, the therapeutic strategy should be reconsidered, because the treatment of the focal infection may be inadequate or the focal infection bears no relation to the PPP.
In addition, treatment of severe constipation or irritable bowel syndrome also improves the symptoms in some cases (refer to What causes PPP?). This treatment rarely results in a cure of PPP, as the condition is often a result of various factors acting in combination. Patients with persistent constipation/diarrhea should consult medical specialists. Regular dietary habits are also important. Many patients have started purchasing and taking Butyric acid based on information from the Internet, etc. However, not all patients need Butyric acid, and no drug therapy is effective in all patients. Butyric acid may cause intestinal gas and/or constipation in some patients. Therefore, Butyric acid intake by patients casually, at their own discretion, is not recommended.
Dysbiosis, which is a change in the bacterial flora, is seen in both tonsillar/focal dental infection and in patients with intestinal symptoms. Many patients may know about intestinal flora, and it appears that many species of commensal bacteria which protect our mucosa coexist to maintain health. It is speculated that bacteria that are little pathogenic, but serve as triggers of excessive immune responses, dominate in the intestinal bacterial flora of patients with repeated diarrhea/constipation, in the periodontal flora in patients with periodontitis, and in the commensal bacterial flora in cases of tonsillar focal infection, etc.
Diabetes mellitus, autoimmune thyroiditis, etc.
Diabetes mellitus 4) is a commonly encountered underlying disease in patients with PPP. It is not rare that PPP is comorbid with autoimmune thyroiditis conditions, such as Basedow’s disease or Hashimoto’s disease 18), and some patients with underlying autoimmune thyroiditis may not completely resolve even after removal of focal infections. Since control of these diseases is often useful for improving the symptoms, patients should seek treatments for these diseases from medical specialists (refer to Comorbidities of palmoplantat pustulosis (PPP)).
Psychiatric disorders and mental stress are also known to exacerbate the symptoms. Patients who have these problems and are unable to solve them by themselves should consult their doctors. Alternatively, they may seek the opinion of a psychosomatic specialist.
In addition, there are diseases whose relationship to PPP is not well known, such as hyperlipidemia and hypertension, and patients must let their doctors know about these diseases as well, since control of these diseases would be desirable when the patient is receiving drug therapy.
Dental metal allergy
Metal allergy is also known to be a trigger factor of PPP, however, we found that only 5% of PPP patients are comorbid with metal allergy18). Treatment for focal infection is usually performed at the same time as metal removal. A retrospective cohort study was recently conducted to investigate which mainly contribute to resolution or improvement of PPP; whether treatment of the focal infection or removal of the allergic metal. It is found that metal removal was only a minor factor19). Even if patch test is positive, and its metal is present in the oral cavity, you should not consider the metal is causative factor for PPP. Taking into consideration of the statistical frequencies, priority should be given to the treatment of focal infection. Since retreatment after placement of expensive dental materials places a heavy economic burden, usually patients can first wear a metal prosthesis covered by health insurance. Even after treatment of focal infections and sever itchy vesicles continues, metal allergy should be considered as cause of PPP at this timing. You should not remove all dental metals automatically. If you have medical certificate by dermatologist, the health insurance may cover the cost of expensive dental materials. In addition, even if the eruptions are related to metal allergy, the allergen (the cause of the metal allergy) is not always dental metals, but may sometimes be metals contained in food. Furthermore, skin biopsy is sometimes required to differentiate PPP from other diseases with similar symptoms.
➢ Detection and treatment of asymptomatic focal infection
Tonsillar focal infection
Tonsillar focal infection is treated by tonsillectomy, which requires hospitalization. The operation is performed by otolaryngologists under a general anesthesia. One to 2 weeks of hospitalization is required, because patients cannot eat after the surgery, and if postoperative bleeding is observed, second operation is needed to stop bleeding. A preoperative test to predict an association with PPP has long been searched for, but unfortunately no such biomarkers have been identified yet. All current tests, such as antistreptolysin O (ASO) test, antistreptokinase (ASK) test, and tonsillar provocation test and annulation tests, are used for recurrent tonsillitis caused by pathogenic group A β-haemolytic streptococci and focal infection, and there are no tests for commensal bacteria.
Then, how can the efficacy of tonsillectomy be predicted before surgery? Although Andrews proposed the existence of a correlation between PPP and focal infection 80 years ago, the underlying pathogenic mechanism remains unclear. However, the statistical evidence has been shown that tonsillectomy leads to a cure or marked improvement of PPP in about 80% of patients. So if treatment for other focal infections such as focal dental infection has also been completed and continue to stop smoking, most of Japanese PPP patients can be cured with tonsillectomy. If such signs as an increase in the number of pustules or exacerbation of joint pain after a common cold or other causes of sore throat, the probability is very high that PPP is cured by tonsillectomy. There is a possibility to quantify the clinical parameters between tonsil and PPP with cooperation of patients. For example, comparison of the number of pustules, and comparison of the quantified severity of pain (pain visual analogue scale [VAS]: the severity of pain is quantified on a 100-mm scale where 100 mm indicates the worst pain) before and after common cold are called as self-assessment scores, which are officially utilized in the medical evaluations.
Focal dental infection
Focal dental infection is the most common cause of PPP in Japanese patients. Focal dental infection is detected in more than 60% of Japanese patients with PPP, and its treatment leads to a cure or marked improvement of PPP in over 75% in almost 1 year after dental treatments, with bacteriostatic antibiotics such as macrolide or minocycline. Studies from multiple institutions have also reported that the treatment of focal dental infection is effective in 60% to 90% of the patients. Detection of focal dental infection is relatively easy. Even if tonsillectomy is performed without treatment of focal dental infection, PPP or PAO may recur.
There are 3 types of focal dental infection: apical lesions in which the bacterial mass is confined to the base of the teeth (apical periodontitis), moderate or severe periodontitis with alveolar bone lysis (marginal periodontitis, so-called pyorrhea), and wisdom tooth periodontitis.

Apical lesions (apical periodontitis) occur at the root of the teeth when dental pulpitis progresses. Bacteria that reach the root of the teeth form abscesses, and apical lesions occur as a result of a biological defense response, in which granulation tissue surrounds and confines the bacteria to prevent their spread, even by allowing jaw osteolysis. Apical lesions tend to occur particularly in pulp-extirpated (the so-called nerve-extracted) teeth, and can sometimes manifest with acute symptoms of gingival swelling and pus. If acute symptoms, such as swelling, pain and pus discharge, are absent, the lesions are not usually treated. On the other hand, even if symptoms are absent, the focal infection can elicit immune responses and cause PPP.
Periodontitis (marginal periodontitis) is represented by so-called pyorrhea. If a dental plaque (bacterial mass) accumulates between a tooth and the gingiva and progresses, osteolysis of the alveolar bone, which supports the teeth, will occur, resulting in deepening of the periodontal pockets.Furthermore, the osteolysis results in failure of the alveolar bone to support the teeth, resulting in loosening of the teeth, and eventually falling of the teeth. Moderate or severe periodontitis with a periodontal pocket depth of more than 4 mm could cause PPP. Sufficient oral hygiene of the third molars (wisdom teeth) is sometimes difficult due to incomplete eruption or inclination, and the third molars in which inflammation is the most likely to occur repeatedly are considered as candidates for extraction.
Apical lesions can be recognized as well-circumscribed transmission images on plain radiographs depicting bone (arrow in the left panel of the figure), because of jaw osteolysis. Therefore, apical lesions can be detected by orthopantomography depicting the entire dentition, or other examinations. However, since plain radiographic images are two-dimensional, even experienced dentists cannot detect some apical lesions, and if the results are unclear, three-dimensional computed tomography (CT) is used to identify apical bone defects. Periodontitis is detected by dentists by measurement of the periodontal pocket depth. If periodontitis progresses, the alveolar bone, which is supposed to be present, is not visible due to osteolysis and the alveolar bone line seems to have receded on orthopantomographic images depicting the entire dentition (arrow in the right panel of the figure).
Since the treatment of dental foci stimulates immune system itself, the risk of worsening of the skin and osteoarticular symptoms would be lower if the dental focal treatment is finished in shouter time. Dentists have their own areas of expertise, and general dentists may refer the patients to a specialist cooperating with him/her. The treatment requires adoption of dental surgical techniques in many cases. Even after consulting a specialist, patients should visit their primary-care dentists for usual dental care.
Since the treatment of dental foci stimulates immune system itself, the risk of worsening of the skin and osteoarticular symptoms would be lower if the dental focal treatment is finished in shouter time. Dentists have their own areas of expertise, and general dentists may refer the patients to a specialist cooperating with him/her. The treatment requires adoption of dental surgical techniques in many cases. Even after consulting a specialist, patients should visit their primary-care dentists for usual dental care.
In the treatment of apical lesions, tooth extraction is not always required. In root canal treatment dental prostheses are used to clean the roots. Apicectomy is available for anterior teeth, because of the lack of alveolar bone. Gingiva is incised to remove the bacteria mass. In some cases, tooth extraction may be necessary in case the tooth has a major crack, or size of tooth is very small, or the tooth is fragile.

In cases where several teeth need extraction or in cases where teeth serving as bridge abutments have apical lesions and the only treatment is tooth extraction, we recommend that you consult with dentist, dermatologist, rheumatologist, and orthopedic surgeon regarding the contents and timing of treatment. You must consider the influence on the masticatory function and severity of PPP or PAO. Without treatment of dental focal infection, immunosuppressive drugs is often ineffective, and even if bacteriostatic antibiotics alleviates the skin and osteoarthritic symptoms temporarily, unfavorable long-term treatment with antibiotics becomes inevitable. Patients must consult with dermatologists or rheumatologists regarding the timing of tooth extraction in the situation where the pain killer does not work nor you have inability of body movement such as difficulty to walk. Also the risk of bone fracture due to progression of spondylitis must be informed to patients by dermatologists or rheumatologists if such symptom exists. Patients should also consult their dental specialists about reconstruction method of the masticatory function after tooth extraction. Eventually, the patients with impaired activities have to make a decision under the consideration on benefit such as recovery of walking capability and disadvantage of tooth extraction.
Sinusitis
Chronic sinusitis (empyema) sometimes causes PPP, and patients with persistent nasal congestion or postnasal drip should consult an otolaryngologist. Sinusitis can also be diagnosed relatively easily. Sinusitis can be divided into common sinusitis, which can be treated by an otolaryngologist, and or odontogenic sinusitis, in which an apical lesion spreads to the maxillary sinus, which is treated by a dentist.
15) Okubo Y. Treatment of palmoplantar pustulosis. PPP frontier 2016; 1:28-32
16) Kobayashi S. Focal infection and palmoplantar pustulosis. Visual Dermatol 2012; 11: 1036-1041
17) Takahara M, et al. Tonsils and palmoplantar pustulosis (from the standpoint of an otolaryngologist). Visual Dermatol 2012; 11: 1042-1047
18) Kobayashi S. Management of pathological conditions that are difficult to treat. (4) Diagnosis and treatment of pustulosis palmaris et plantaris. Rinsho Derma (Tokyo) 2018; 60: 1539-1544
19) Masui Y, et al. Dental metal allergy is not the main cause of palmoplantar pustulosis. J Eur Acad Dermatol Venereol 2019; Jan 17. doi: 10.1111/jdv.15434
20) Ueda Y, Shiota S: Clinical Studies on the dental focal infection – interrelationship of a skin disease: pustulosis palmaris et plantaris. Jpn J Oral Diag/Oral Med 1991; 4: 464-468.
➢ Drug therapy and Phototherapy (Symptomatic therapy)
Symptomatic therapy refers to treatment intended to alleviate the symptoms. Drug therapy includes topical therapy (ointments), oral therapy (oral drugs), and treatment with biologics (injection). In addition, phototherapy (UV light therapy) or granulocytapheresis (granulocyte and monocyte adsorption apheresis (GCAP)) aimed mainly at the relief of osteoarthritic pain is sometimes used concurrently. These treatments will usually not be fully effective, if the obvious cause of the disease, such as focal infection, is left untreated. For safe and effective treatment, appropriate treatments should be selected and combined after fully considering whether the factors causing the disease will be removed by the treatment and how long the treatment would last. Patients should undertake an in depth discussion with their doctor to arrive at a decision.
1)Topical therapy (ointments)
Topical therapy refers to basic symptomatic therapy and is actually very important. Patients should correctly understand not only which ointment should be applied, but also how the ointment should be applied.
●Types of ointments
Ointments include “topical steroid” ointments, which is fast acting and suppresses immune responses to control inflammation, and “topical vitamin D3,” which gradually suppresses the proliferation of the skin cells and reduces pustules.
Topical steroids
Topical steroids relieve inflammation by suppressing immune responses and causing localized vasoconstriction. Topical steroids vary in strength. Use of too strong topical steroid sometimes oversuppress regeneration of epidermal cells, and on the palms and soles, the stratum corneum will become stiff and crack. Or, recovery of the skin could become delayed after desquamation of the horny layer, causing the skin to become too thin. Use of a very weak topical steroid, on the other hand, may not be fully effective. Patients should follow the instructions of their dermatologists and immediately let the dermatologists kno of their concerns, if any.
Topical vitamin D3 analogues
In cases of PPP, topical vitamin D3 is effective for relieving the vesicles and pustules and suppressing the development of new pustules. Topical vitamin D3 also suppresses and normalizes the proliferation of epidermal cells by a mechanism different from that of steroids. In some patients, desquamation sometimes happens far beyond the area of application of topical vitamin D3. If the use of topical vitamin D3 expands the lesion, patients should consult their doctors. These problems could be prevented if the topical vitamin D3 treatment is only used when vesicles and pustules appear and is immediately stopped when the vesicles and pustules erupt.
*Combination products of steroid plus vitamin D3
This combination products are often used for topical treatment of psoriasis and is advantageous in that the effects of both drugs can be obtained at the same time. However, at present, there is no combined steroid/vitamin D3 drug preparation that is covered by health insurance for the treatment of PPP. In addition, the combination drug has the advantages and adverse reactions of both drugs, and if improved efficacy of only one of the two component drugs is desired, no adjustments can be made; for example, even if the symptoms are improving, a slightly stronger steroid may need to be applied. Caution should be exercised to avoid skin atrophy due to topical use of steroids. the lesion with lint slathered with zinc oxide ointment. This treatment has often allowed patients who walked into a hospital limping with pain to walk back smoothly to their homes.
Since this is an oily ointment, it tends to stick to clothes and floors and thereby dirty the surroundings. Therefore, patients should carefully learn from nurses about how to handle this ointment. The ointment can be easily removed using unpressed absorbent cotton impregnated with olive oil. It does not need to be completely removed. Even if a small amount of the ointment is left behind, the wetness should be removed after cleaning with soap and the ointment should be applied over it. Since the skin does not dry out, there is no pain. Skin turnover (renewal) is rapid in the skin with inflammation, and if the skin improves, the horny layer with the ointment will peel off.
Zinc oxide ointment
Zinc oxide ointment, one of the basic topical treatment agents used in dermatology, is a pure white oily ointment often used also for diaper rash in babies. It has an excellent skin protective effect and is extremely effective for improving oozing wet conditions or when desquamation or cracking of the horny layer of the skin causes pain. It also has anti-inflammatory effects. Here is how to use. First, a topical steroid, topical vitamin D3, petrolatum, or other ointment is applied, followed by coverage of
Video (How to use zinc oxide ointment)
Video (preparation of zinc oxide ointment: ointment is applied to lint and stored)
Skin moisturization and therapeutic agents for keratosis
Moisturization and protection of the skin are very important. Read carefully the following tips for topical therapy.
Moisturization of the skin helps the skin to recover normally. Dry and rough skin interferes with smooth repair of the skin. Ointment should be applied repeatedly, as necessary, to maintain a moist condition of the skin. Humectants include hydrophilic ointments, heparinoid-containing ointment and petrolatum/vaseline, as well as urea ointment, which is effective for moisturization of the skin and dissolution of the stratum corneum. In addition, if the stratum corneum is too thick, use of salicylic acid ointment to soften the horny layer would prevent from cracking.
Use of Tubifast® (tubular bandage), which was developed for ointment therapy, serves a dual purpose: it prevents stickiness immediately after application of the ointment and prevents drying. If the ointment seeps through, two layers of Tubifast® should be used. Tubifast® can be washed and reused a few times.
 Tubifast ®
Tubifast ®
●Tips for topical therapy
Important tips for topical therapy are: do not rub ointment and do not scratch, nor remove nor trim the stratum corneum that stated to peel off!
In topical therapy for PPP, it is most important not to rub ointment and not to scratch, nor remove nor trim the stratum corneum (scales and desquamation) that started to peel off. Almost all patients remove or trim the scales, then apply the ointment. Also, many patients tend to peel off scales. However, those conducts serve as stimuli, triggering the development of new pustules and promotion of inflammation.
Since the skin of the palms and soles has a thick horny layer and its recovery takes time, after separation of the pustules or scabs, the stratum corneum begins to gradually peel off and skin repair occurs slowly. Patients feel very uncomfortable and worry that ointment application on the thick horny layer or peeling off scales is not effective. However, they should not worry. Ointment will be absorbed, so patients should not be distracted by scales and just apply the ointments quickly. As occlusion therapy, cover the area with zinc oxide ointment, humectants, Tubifast®, a glove, etc.
Why should patients not touch the lesions unnecessarily? This is because the horny layer contains in abundance substances that recruit neutrophils which consist of pustules. In addition, the epidermis with vesicles also contains an abundance of cytokines that can cause inflammation. By stimulating these horny layer and epidermal cells, inflammatory substances are released, and pustules are aggravated and lesions expand.

Mechanism of pustule formation; Figure modified from Ozawa M21), Murakami M22)
21) Ozawa M, et al. Localization of IL-8 and complement components in lesional skin of psoriasis vulgaris and pustulosis palmaris et plantaris. Dermatology 2005;211(3):249-55
22) Murakami M, et al. Vesicular LL-37 contributes to inflammation of the lesional skin of palmoplantar pustulosis. PLoS One. 2014 Oct 16;9(10):e110677
2)Phototherapy (UV light therapy)
Phototherapy, UV light irradiation, has a long history, and is used for various chronic skin diseases. UV light irradiation reduces the number of activated lymphocytes, inactivates inflammatory molecules, and induces regulatory T cells, which suppress the inflammation. It is effective only for skin symptoms. Phototherapy is a symptomatic treatment, and is used during causal treatments, or with patients who have no triggers of PPP. The patients need to visit the hospital frequently, e.g., once a week. UV light therapy can be undertaken at nearby clinic of the patients by collaborating with the attending doctor.
UV light therapy includes PUVA therapy (psoralen-UV-A irradiation) and narrow-band UVB therapy using selective ultraviolet B. For narrow-band UVB, TL-01, TARNAB®, excimer light, which have slightly different wavelength and peaks, are available. Any of them can be used.

PUVA therapy for the hands and feet:
Apply psoralen (an enhancer the response to UV light) on the lesion, then the skin is irradiated with UVA. As the effect of psoralens persists, psoralen should be fully washed off with soap, and avoid sunlight at least for 12 hours.
Various narrow-band UVB irradiation devices (examples available in Japan)

Full body type (TL-01)

Excimer light(TARNAB®)
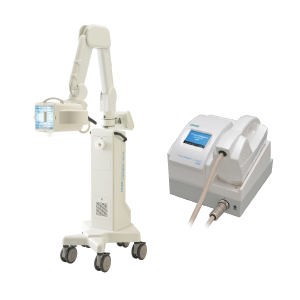

Excimer light (left:therapyIm®, right:VTRAC®)

3)Oral therapy
―Drugs used to alleviate skin symptoms―
1) Biotin /Butyric acid /Ascorbic acid
Biotin therapy i.e. high dose of biotin, butyric acid and ascorbic acid have sometimes been used. However, unless you conduct causative therapy such as treatment of focal infection and smoking cessation, biotin therapy itself is ineffective.
To date, there is little scientific evidence to support the biotin therapy. After causative therapy, high dosage of biotin sometimes alleviates the skin symptoms. However, animal experiments suggest that high dosage of biotin influences development and growth of fetus, so any females desiring to have a baby are not recommended to take this therapy. In addition, since high dose biotin also relieves severe pain caused by pusturotic arthro-osteitis (PAO), it tends to be misunderstood as being effective for joint symptoms, and in some cases, long-term suppression of pain alone prevents the patients from being aware of disease progression, resulting in the patients gradually developing severe spondylitis. Likewise, PAO is also very often caused by focal infection, and priority should be given to the treatment of focal infection.
A Butyric acid should be tried in patients with persistent diarrhea/constipation. Oral butyric acid can improve colonocyte regulation and bacterial microflora composition by exerting anti-inflammatory activity in intestine, and in some occasion helped to improve the skin eruptions in some patients with PPP. However, butyric acid is not effective in all patients with PPP. In fact, some patients develop diarrhea and/or abdominal distension after taking butyric acid. Thus, there are differences among individual PPP patients in the significance of the intestinal bacterial microflora. It is considered to be ideal for the intestinal bacterial microbiota to comprise a wide variety. It is well known that intestinal flora is called “intestinal microflora,” by reference to the phrase “garden of flowers.” It is necessary to study the relationship between PPP and bowel symptoms/characteristics of the intestinal microbiota in Japanese patients as well. Patients should also try and keep regular dietary habits, and if the intestinal symptoms do not improve, a medical specialist should be consulted.
2)Antihistamines and Chinese herbal medicines
Antihistamines can be concurrently used to control itching. Among Chinese herbal medicines, Jumi-haidoku-to and Hainou-sankyu-to, that suppresses pus formation, Oren-gedoku-to, that is effective for dermatitis with itching, Shofu-san, that is effective for itching and in retaining skin moisture, and Keigai-rengyo-to, that is useful in the presence of underlying tonsillitis, and other medicines can be tried.
3) Vitamin A derivatives (Etretinate)
Etretinate is an oral drug for the treatment of PPP that is covered by health insurance in Japan. Vitamin A derivatives suppress abnormal proliferation of the skin epidermal cells, regulate skin metabolism, and suppress inflammation, preventing the formation of pustules. However, etretinate is contraindicated in pregnant females. Therefore, it is not recommended for people of childbearing age, and its use can only be considered in postmenopausal females and males whose partners are postmenopausal. It is known to exert synergistic effect when combined with UV light therapy. However, it should be used carefully, by starting with a low dose, because it has adverse reactions such as liver dysfunction and higher doses may cause more severe skin and mucosal dryness and irritation, hair loss and nail deformities. Furthermore, since its long-term use can cause ossification of large joints, it is not actively used for treating PAO.
―Drugs expected to be effective against both skin symptoms and arthro-osteitis―
1) Cyclosporine (note: its use for PPP is not covered by Japanese health insurance)
Although its use is not covered by health insurance in Japan, cyclosporine is effective against the skin symptoms and particular types of PAO, such as sternoclavicular arthritis with chest pain and peripheral arthritis of the hands and feet with PPP patients. Caution should be exercised against the development of hypertension and renal impairment as adverse reactions to cyclosporine in patients receiving the drug. In particular, since cyclosporine is known to cause renal vascular sclerosis, it is desirable to use cyclosporine for less than 2 years and its use should be avoided with patients aged 65 years or older in whom arteriosclerosis tends to be advanced. On the other hand, in younger patients,adverse reactions do not tend to occur, and cyclosporine can be used while confirming its safety by regular blood tests, urinalysis and blood pressure measurement.
2)Antimicrobials
Antimicrobials are used to treat focal infections, in principle, but they can also sometimes be used to alleviate the skin/osteoarticular symptoms. Use of antimicrobials for the treatment of chronic tonsillitis, chronic periodontitis, chronic sinusitis and other diseases is covered by health insurance. However, in cases with an obvious focal infection, for preventing long-term use of antimicrobials, it is important to prioritize the treatment of focal infections.
3) Apremilast (note: under clinical study as of 2019)
Apremilast is a type of disease-modifying anti-rheumatic drug (DMARD) which inhibits Phosphodiesterase-4 (PDE4). It works by targeting PDE4 involved in the inflammatory processes. Apremilast causes side effects such as diarrhea, nausea, headache, and feelings of depression and suicidal thoughts. In those cases, contact your doctor immediately. Weight loss is also observed in some patients.
―Drugs for osteoarticular symptoms― (also refer to: Treatment of PAO)
1) Painkillers (non-steroidal anti-inflammatory drugs: NSAIDs)
Painkillers are used for pain control with treatments of focal infections. It may cause side effects such as gastritis, gastric ulcer and/or renal impairment by long-term use of NSAIDs.
2) Bisphosphonates
Bisphosphonates, used to treat osteoporosis, are also effective, to some extent, against sternoclavicular arthritis with residual pain, after removal of trigger factors.
3) Corticosteroids
Corticosteroids are sometimes administrated orally for one or two weeks or injected into the joints with severe pain patients just as an emergent treatment.
4) Disease-modifying antirheumatic drugs (DMARDs)
Methotrexate, which is used for rheumatic arthritis, may also be used for peripheral arthritis in PAO. Salazosulfapyridine may be used for axial arthritis, such as spondylitis or sacroiliitis.
5) Others
Chinese herbal medicines such as Makyo-yokukan-to, Dai-bofu-to, or colchicine also used for PAO.
4) Biologics (injection)
Inflammatory substances called cytokines are involved in the development of PPP. Cytokines are proteins produced by the white blood cells and other cells in response to various stimuli and induce transmission of inflammatory signals to other immune cells. In PPP, cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-23, IL-17 and IL-8 are thought to be complicatedly involved in the development of the symptoms. Although all of them are important cytokines involved in the body’s immune responses, persistent excessive production of these cytokines results in progression of the disease to a chronic inflammatory diseases.
Biologics are artificially produced antibodies (proteins) by most recent biotechnology that bind to and inactivate specific cytokines. In 2018, biologics targeting IL-23, guselkumab (Tremfya®) was approved also for the treatment of PPP in Japan. Currently more new biologics are under development in Japan as well as other countries.

Biologics are indicated for moderate to severe PPP patients who are refractory to conventional treatments. There is approval system for treatment of biologics. The medical institutions must conduct cooperation with radiology and respiratory medicine, enabling early detection and treatment of adverse reactions (https://www.dermatol.or.jp/modules/biologics/index.php?content_id=4).
In addition, TNF-α inhibitors have been also used particularly for patients with severe PAO, although this agent is not covered for PPP by health insurance in Japan. It should be recognized that TNF-α inhibitors may exacerbate PPP skin lesions itself. In addition, IL-17A inhibitors have been shown to be effective in some cases with PAO. However, IL-17A is an important cytokine which control antimicrobial peptides, so careful attention must be made whether this treatment can be done safely without exacerbating focal infections.
5) Granulocytapheresis (G-CAP)
Granulocytapheresis (G-CAP) is a treatment for pustular psoriasis, but sometimes PPP is considered as a localized form of pustular psoriasis, and granulocytapheresis is used in combination with other treatments, when the joint symptoms cannot be controlled. G-CAP removes activated white blood cells (granulocytes and monocytes) from the blood, resulting in reduction of inflammation. Blood is pumped from one arm and passed through a column that absorbs only activated white cells, and the remaining blood is returned to the body through the other arm. It reminds you of dialysis. However, unlike in dialysis, water contents and waste products are not removed in G-CAP, therefore, it gives almost no burden on your body. Since approximately 20% of all the blood can be treated at a time, a standard course consists of 5 treatment sessions at weekly intervals.
*High-cost medical care benefit system in Japan
Please refer to the website of the Ministry of Health, Labour and Welfare. Also please consult with your health insurance carrier.
https://www.mhlw.go.jp/bunya/iryouhoken/iryouhoken01/dl/01_eng.pdf
●Advice in your daily life
1.Stop smoking
Smoking is considered to trigger the development/aggravate PPP. Let’s challenge to stop smoking. Smoking cessation clinic is also effective.
2.Try to prevent/treat early infections such as common cold
When you catch a cold, inflammation occurs in tonsil or pharynx, or sinusitis may also relapse. These trigger factors exacerbate PPP. Prevention and early treatments of cold is strongly recommended. Gargling and hand-washing are effective methods.
3.Oral care
In order to avoid relapse of periodontitis, let’s keep oral environment clean. Periodical dental cleaning also recommended.
4.Try to avoid physical stress on the sites of arthro-osteitis
Even after improvement of joint and bone pain and inflammation, the symptoms may relapse by placing physical stress such as carrying the heavy goods, wiping the windows, or snow plowing, or doing excessive workouts, etc.
5.Control mental stress
PPP is related to the immune system disorder. The symptom tends to worsen by mental stress. Let’s review your mental situation if you have an excessive stress or not. Let’s forget stressful things. Tomorrow is another day.
6.Reconsider what to eat to improve the intestinal environment
Severe constipation or diarrhea may exacerbate the symptoms. Try to cultivate regular dietary habits to improve the intestinal environment. Don’t easily take the supplements or foods which claims to clean the intestine environment because some of them may not adapt to yourself. We recommend regulated dietary practice.
Concluding remarks
Treatment of PPP is considered to be difficult, because the disease varies in nature/severity among patients. Although PPP is primarily a disease of the skin and bone/joints, trigger factors for the disease frequently reside in different organs from disease sites, making it difficult to understand the precise pathogenesis of this disease.
However, PPP can be cured. If there is a treatment that can lead to cure or remission, it will be ideal to cure the disease before osteoarticular lesions become chronic. Many patients say that they did not expect that even their bones would be affected, despite having only mild skin symptoms at first. In order to choose the appropriate treatment(s) without regret, and not to miss the timing of appropriate treatment, we recommend Japanese patients to learn about Japanese type of PPP and PAO in depth. It is very important for the patients themselves to be actively involved in determining the therapeutic strategy, so that they can help their doctors develop a suitable treatment plan and to proceed with treatment while modifying it as needed.
Satomi Kobayashi

