About Palmoplantar Pustulosis
Palmoplantar Pustulosis
➢ What is palmoplantar pustulosis (PPP)?
Palmoplantar pustulosis (PPP) is a type of skin disease that is characterized by repeated eruptions of multiple small pustules on the palms of the hands and soles of the feet. Although these small vesicles are filled with pus, the pus is sterile (it is “sterile” because no infection is observed).
Since patients often feel an itching sensation when the blisters are forming, it may be misdiagnosed at first as roughness of the hands or athlete’s foot. However, topical treatment often fails to alleviate the symptoms.
As the pus-filled vesicles form repeatedly on the skin, the affected area of the skin also expands.
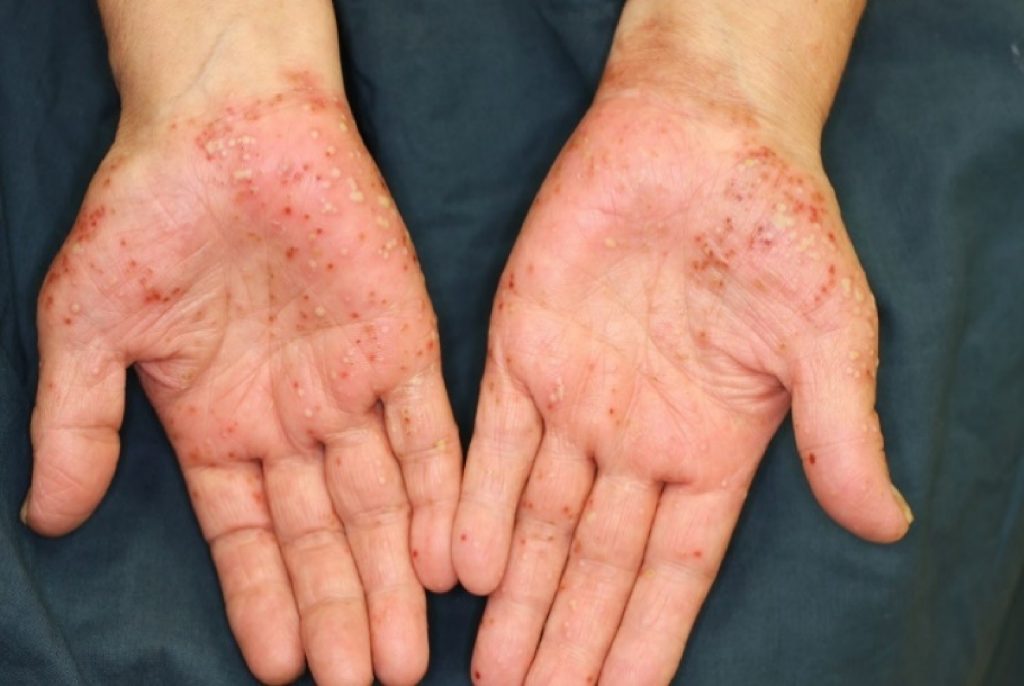
Pustules (white), vesicles and scab (reddish brown) on the palm
The disease is not contagious. Since the pus is sterile, the disease is not transmissible by contact. Due to the poor awareness of the general population of PPP as a non-contagious disease, there could be a stigma attached to the disease, and people may avoid coming in contact with patients with PPP for fear of becoming infected. Thus, patients with PPP are sometimes hurt by the thoughtless remarks and inconsiderate behaviors of others. Some patients always wear gloves, do not go to pools or hot springs, and feel depressed on account of fear of such rejections.
Some patients are unable to walk or use their hands due to pain, and some others reveal acute severe chest pain. If there are multiple pustules on the palms of the hands and soles of the feet, it can significantly impair the patient activities of daily living (including inability to walk due to pain, difficulty with washing face/hair, grocery shopping, cooking, etc.). The disease causes significant deterioration of health-related quality of life (HR-QOL).
Furthermore, 10-30% of patients also manifest inflammation of the clavicle, sternum, spine. In some cases, the patients suffer from sudden onset of severe chest pain, which could be mistaken for angina pectoris or myocardial infarction.
<Contents>
➢ Palmoplantar pustulosis – Skin and nail symptoms
➢ Joint and bone symptoms – Pustulotic arthro-osteitis (PAO) associated with palmoplantar pustulosis
➢ What causes palmoplantar pustulosis (PPP)?
➢ For Comorbidities
➢ Is it true that Japanese patients are more susceptible to palmoplantar pustulosis?
➢ Which symptom will be observed first, skin pustules or bone/joint pain?
➢ Is it curable?
➢ Which clinical department should I go?
Palmoplantar pustulosis (PPP) – Skin and nail symptoms
The skin symptoms of PPP include 1) pustules on the palms of the hands and soles of the feet (planta), 2) eruptions in areas other than the hands and feet, and3) nail symptoms.
Symptoms of hands and feet
A characteristic symptom of PPP is the development of pustules, consisting of small vesicles on the palms of the hands and soles of the feet (planta).
Although pustules on the hands and feet can develop in other conditions, the pustules of PPP have characteristic features.
In the Japanese type of PPP, each pustule of PPP is formed in two steps: A small vesicle is formed first, and white pus emerges from the center of the vesicle. Although all inflammation is caused by white blood cells (probably monocytes), the white blood cells responsible for the initial inflammation are different from those responsible for the white pustules (neutrophils) (refer to What causes palmoplamtar pustulosis (PPP) and Pathogenesis on palmoplantar pustulosis).
Since the vesicles are very small, patients often detect them only when they feel the itching sensation during the formation of the vesicles. A short time after a vesicle becomes a white pustule, it becomes dry, followed by the formation of a brownish scab, which is easily noticeable.
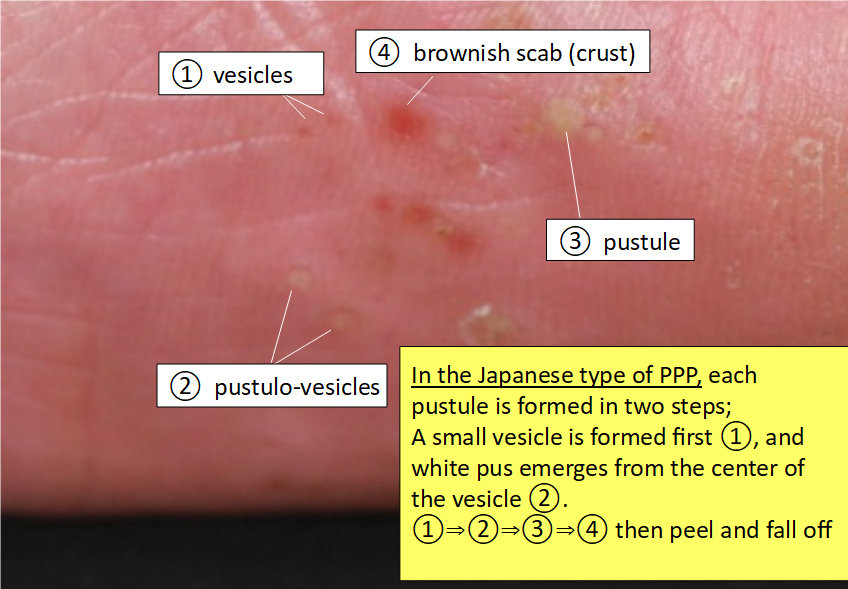
Cited and modified from the article by Satomi Kobayashi, Rinsho Derma, Vol. 60 (2018) (*3)
After a few days, as the skin underneath the lesion starts healing, the skin lesion along with the surrounding stratum corneum (white crust called “scale”) peel off spontaneously and fall off. The area of the affected skin then looks red (erythema) and dry.
After a few repetitions of the same process on the palms of the hands and soles of the feet, a diffuse area of the skin turns red and dry. The palms of the hands and soles of the feet look red, covered with pustules, scabs and peeling off skin.
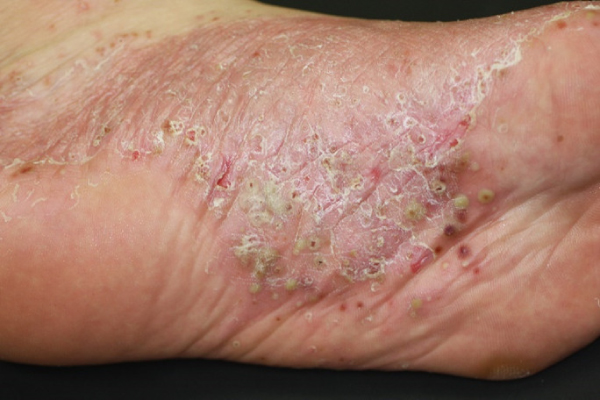
When many layers of the stratum corneum pile up, the skin loses its elasticity and cracks. Cracking of the skin causes pain. If the entire soles of the feet become covered with skin eruptions, it becomes impossible to walk due to pain. Some patients have described feeling as if they are walking on broken glass shards or pushpins.
You need to monitor frequency of pustule formation
In the Japanese type of PPP, when new pustules form continuously, it is necessary to find out if there is any hidden trigger factor for the PPP, for example, focal infections such as tonsillar lesions or odontogenic focal infection. (refer to “What causes palmoplantar pustulosis (PPP)?” / “Treatment for PPP”).
Attention should be paid to any discomfort or swelling in the throat or gingiva when you are tired and to possible aggravation of symptoms with a cold. If you notice any such condition, you need to inform your attending physician.
The skin symptoms may not improve for decades with only topical treatment. Even if the skin symptoms do improve after high doses of oral biotin for many years, PPP can subsequently progress to bone or joint symptoms if a hidden lesion or focal infection is left untreated.
Smoking can be one of the major trigger factors for the onset of PPP, even the non-Japanese type.
In cases of PPP, pustules often form in a recurrent and periodic fashion. This is presumably attributable to the interaction between the immune reactions causing pustule formation and the immune reactions directed at suppressing the pustule formation.
The pustules often relapse and disappear in cycles of approximately 4 weeks. Even during the period of a single cycle, trigger factors (such as tonsillar or odontogenic infection, or chronic infections including sinusitis) are frequently observed in many cases with the Japanese type of PPP. When the cycle is shorter (for example, pustules relapsing every 1-2 weeks), you need to consult a physician for a thorough search of any asymptomatic focal infection.
If a trigger factor is identified, its treatment or removal usually results in improvement of the lesions within 6 months or so. In 60-80% of patients with the Japanese type of PPP, the skin symptoms usually resolve completely within 1-2 years.
Are you psychologically stressed or overworked?
Immune status also affects the onset/course of the pustules in cases of PPP.
When a person experiences significant psychological stress or pushes him/herself physically (overwork), the number of pustules tends to increase.
Focal infection in cases of PPP is often caused by the commensal bacteria in the nose, throat or mouth (teeth). The normal flora are usually non-pathogenic, coexisting harmlessly with us. When the immune system becomes unstable because of excessive stress or fatigue, however, autoinflammation reacts with dysbiosis and may trigger an excessive immune response (breakdown of immune tolerance).
Palmoplantar pustulosis aggravates with touching or rubbing
Peeling and dried skin (scales) is not only annoying, but can also cause pain. Dry and stiff scales can irritate the skin when a person is standing or walking. You may want to peel off and smoothen the skin to avoid such irritation. However, since PPP aggravates with such mechanical stimuli (Köebner phenomenon), such a tendency should be avoided (refer to “Treatment for PPP”).
The stratum corneum of the palms and soles is significantly thicker than that of other parts of the skin, and takes a longer time to repair. Rubbing and peeling of the skin interfere with return of the skin to a flat and smooth condition. Therefore, the urge to peel of scales should be resisted, and one must wait for the skin to spontaneously heal. You can prevent drying of the skin and keep the skin moisturized with ointments. Zinc oxide ointment can protect the skin from irritation. Rubbing an ointment into the skin must also be avoided (refer to “topical treatment”).
❐ Skin symptoms in areas other than the hands and feet – extra-palmoplantar eruptions –
In some cases, erythematous areas may appear on the elbows, knees, forearms and lower legs. Although the erythematous areas may resemble those of psoriasis, they have less well defined borders and show milder induration as compared to the lesions of psoriasis in Japanese patients.
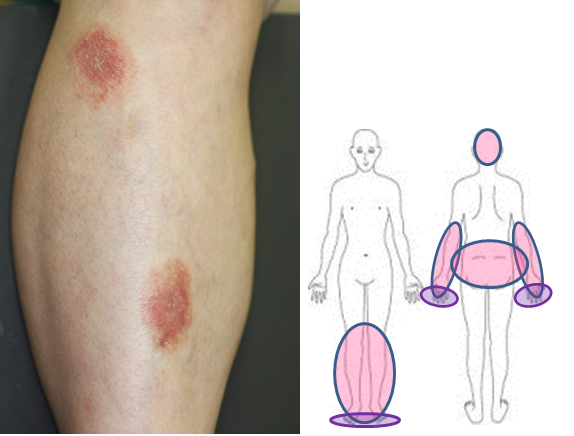
Exstra- palmoplantar eruptions and their distribution
These eruptions are also different from those of psoriasis histopathologically, and are called extra-palmoplantar eruptions. Extra-palmoplantar eruptions can be observed even on the scalp and buttocks, and might be mistaken for eczema and folliculitis in the case of lesions on the scalp or for tinea in the case of lesions on the buttocks. These lesions are often itchy. Since information on the clinical condition will help in the diagnosis (i.e., persisting for a few months and not responding to topical treatment), we strongly recommend that you provide accurate information to your attending physician.
Nail symptoms
Nail manifestations include pustulation under the nail plate, pitting, destructive changes, thickening of the nail or of the skin under the nail plate, and nail discoloration. The changes may lead to nail detachment.
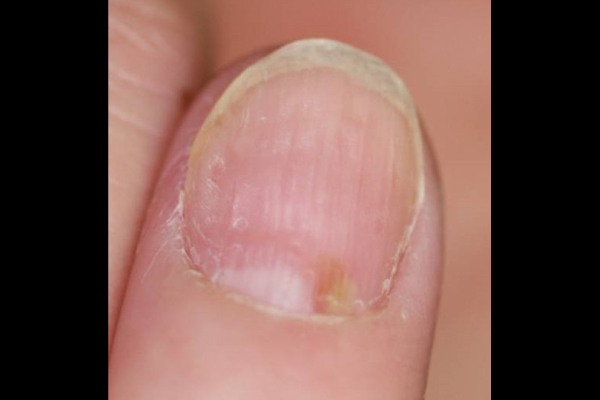
Pustules under nail plate
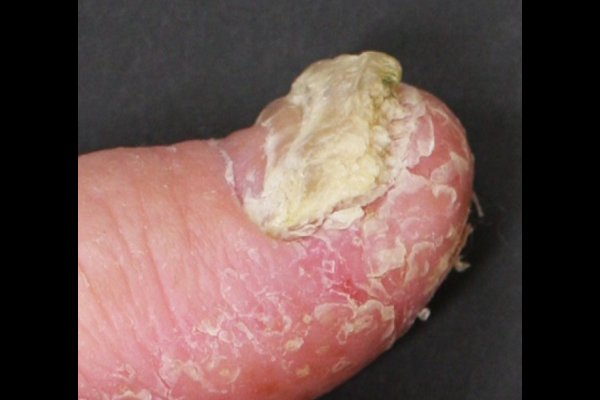
Thickening of nail plate
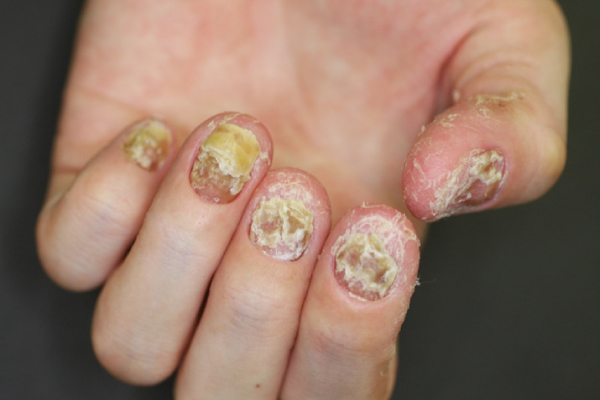
Difficulty in performing tasks using the fingertips due to pain associated with destructive changes of the nails.
Nails are normally visible to everyone, and the aforementioned nail manifestations can lead to depression. Furthermore, broken nails can catch the hairs or clothing fabrics. When the nails are destroyed, it is difficult to pick up things like thin coins. Thus, the nail manifestations can lead to significant impairment of the daily living activities.
These manifestations can improve with appropriate treatments. The attending physician should be informed of all the problems and inconveniences, and should be consulted about the treatments that are available.
Most outpatient dermatology clinics/hospitals are very busy, and a patient may feel hesitant to ask such questions. In such cases, the problems can be explained to the nurses. The attending physician may be given a list of the matters of concern to be discussed at a subsequent visit. This will give the physician an opportunity to consider treatment options appropriate for individual patients.
PPP is curable
PPP is curable. It is important to make a correct diagnosis, promptly identify trigger factors for onset/aggravation, remove the trigger factors and then administer appropriate treatment.
After an aggravating factor is removed, the pustules gradually stop forming over a period of half year to 1-2 years. PPP is often cured in such cases (refer to “Treatment of PPP”).
Joint and bone symptoms – Pustulotic arthro-osteitis (PAO) in SAPHO syndrome, associated with palmoplantar pustulosis (PPP)
About 10-30% of patients with PPP concurrently develop “pustulotic arthro-osteitis (PAO)”. PAO refers to inflammation at the sites of attachment between the bones and tendons or ligaments (enthesitis), and bone itself (osteitis).
Such inflammation manifests as severe pain. It often occurs concomitantly with pustules of the hands and feet, but may occur even after the skin symptoms have resolved.
PAO precedes the appearance of pustules on the palms and soles in some patients, and it could be difficult to make a precise diagnosis in these cases.
Characteristic sternoclavicular arthritis
The sternum, clavicles, joints between the sternum and the first rib, and the rib junctions on the upper and lower sternum are considered as characteristic sites of joint involvement in PAO. About 80% of patients with PAO manifest bone lesions in the anterior chest wall.
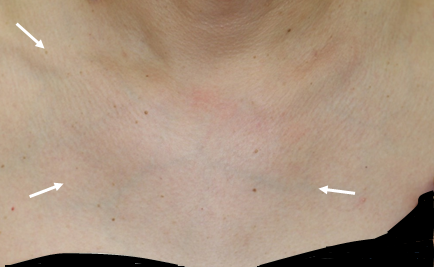
Chest wall swelling is observed
Since the patients may develop sudden severe pain around the neck to the chest, the condition is sometimes misdiagnosed as angina pectoris, myocardial infarction or reflux esophagitis.
The sternoclavicular region moves with breathing. Therefore, even breathing can be painful, and coughing and sneezing entail great pain.
Turning over in bed may cause significant pain. Therefore, many patients have a problem sleeping on their beds, and often doze while leaning against a couch.
Even X-rays may not confirm the diagnosis, because the bone changes of osteoarthritis do not occur immediately.
❐ Pustulotic arthro-osteitis: PAO and SAPHO syndrome
PAO is also sometimes called the Synovitis, Acne, Pustulosis, Hyperostosis, Osteitis (SAPHO) syndrome. The SAPHO syndrome covers approximately 50 terms of diseases, including PAO, acne spondyloarthritis, chronic recurrent multifocal osteomyelitis (CRMO), diffuse sclerosing osteomyelitis of mandible, enteropathic spondyloarthritis. PAO accounts for most cases of SAPHO syndrome in Japanese patients, and has been reported to account for 52-56% of cases of SAPHO syndrome in the world.
However, the underlying PPP has a characteristic feature, that of focal infection as the trigger of the onset of the disease in 60~80% of Japanese patients, which is directly related to the treatment strategy of PAO. In order to stay aware of the importance of eliminating the trigger in the treatment, it is important to treat PAO individually, without considering it under the umbrella of SAPHO syndrome.
The therapeutic approach to SAPHO syndrome also remains unsatisfactory. By analyzing the diseases individually, it may become clear which treatment must be prioritized .

Sited from Depasquale R, et al. SAPHO: What radiologists should know. Clin Radiology 67, 2012
❐ Attention must be paid to pain in the neck, back and lower back
PAO can involves various areas. Inflammation can occur in the neck, spine spine (spondylitis, arthritis of sacroiliac joints), shoulders (arthritis of acromial joints) and hip joints, as well as in the bones and joints of the hands and feet (peripheral arthritis).
The spine bears much of the body weight, and weakening of the spine due to inflammation may cause spinal fractures. Therefore, patients should not take it lightly as mere back pain, but inform their attending physician.
If pain is felt in the soles or heels of the feet, it may be attributed to enthesitis in the major tendons or ligaments that are continuously used on a daily basis. Enthesitis is an inflammation of the entheses (the sites of insertion of tendons and ligaments into bones).
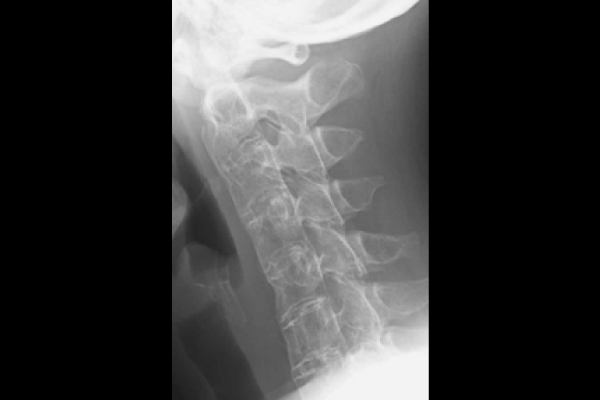
Bamboo spine of the neck
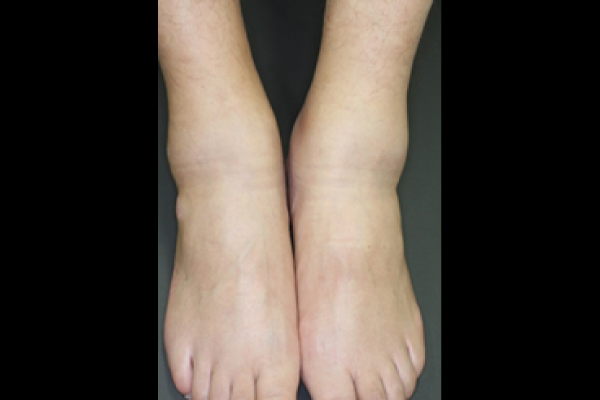
Peripheral arthritis. Swelling are observed on the feet.
Furthermore, osteitis due to PPP can also be observed in the bones of the long bones in extremities (aseptic osteomyelitis).
Bone inflammation is normally accompanied by severe pain.
In such cases, accurate diagnosis is very important. It is necessary to differentiate the pain from that caused by bone metastasis (metastatic cancer) and bone infections due to conventional bacteria or other bacteria such as Mycobacterium tuberculosis.
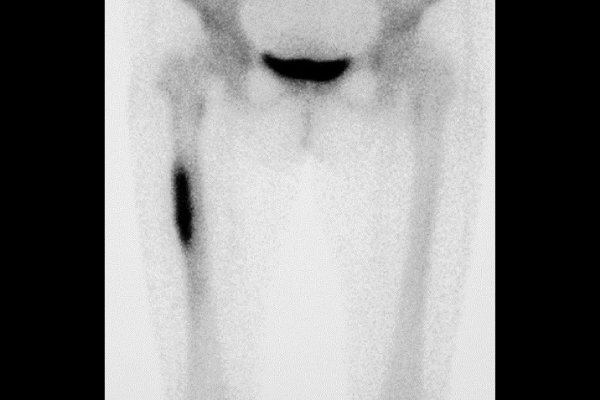
Bone scintigraphy. Inflammation is observed in femur.
What types of examinations should be performed?
There are several examinations for osteoarthritis. The following examinations should be performed on an as-needed basis in order to make a diagnosis, study the current status of inflammation, and determine whether or not the condition is complicated by other bone diseases causing pain. Based on findings of the examinations, a comprehensive judgement is made on what kind of treatment is necessary.
Laboratory examination (blood test)
Acute- and chronic- phase reactants are measured for evaluation of the inflammation. Laboratory examinations are also performed in order to determine the presence of autoantibodies, including rheumatoid factor and antinuclear antibody for differential diagnosis, and to search for comorbidities, including autoimmune thyroiditis, diabetes, hyperlipidemia and other immunological disorders.
Plain radiography
Plain X-rays are the standard radiographs for bones. The types of bone changes can be evaluated, and any cause of lower back pain other than the normal aging process is searched for.
MRI STIR sequence/fat suppressive image
Magnetic resonance imaging (MRI) is the most effective method to identify the bone lesions of PAO. A short tau inversion recovery (STIR) sequence or fat suppressive T2W1 image will not only show bone inflammation as accurately as bone scintigraphy, but also provide further pathoanatomical details. T1W1 imaging will show bone changes of inflammation, such as bone erosion and bone proliferation, and signs of past inflammation such as fat deposition in the bone or ankylosing changes. T2W1 imaging is also used to detect inflammation, however, STIR or fat suppressive imaging is needed to detect PAO lesions. In addition, MRI has the advantage of not exposing a patient to any inherent radiation risk.
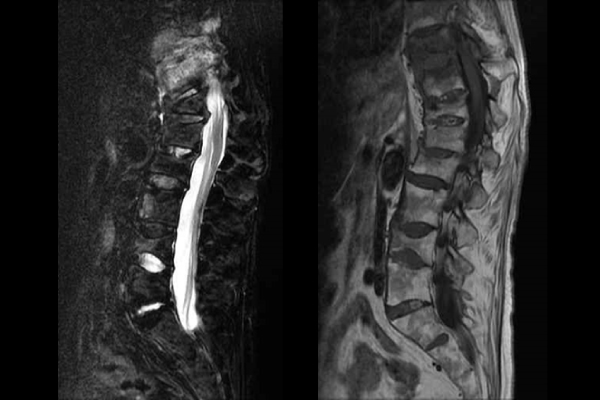
Left: fat suppressive image. Inflammation sites are edematous and looks whitish.
Right: T1W1 image. Vertebral bones show constructive fracture in multiple locations.
- Bone scintigraphy
- In patients presenting with pain in various parts of the body, it is often difficult to find the site of inflammation. Bone scintigraphy, however, allows all sites of bone inflammation in the body to be identified at the same time. Bone scintigraphy does not help in identifying changes in the forms/shapes of bones.
Pulse-echo ultrasound
In arthritis of the wrist, ankle and elbow joints, pulse-echo ultrasound shows vascular proliferation during the early phase of inflammation. Since swelling and bone proliferation due to inflammation can be monitored without radiation exposure, it is a safe and convenient clinical tool.
Characteristics of pain: Pain at beginning of body movement, called “pain at rest”
A patient feels the most severe pain when she/he starts moving the body after resting for a prolonged period of time. This is called “pain at rest,” a characteristic of inflammatory pain.
Examples of “pain at rest” include inability to get out of the bed or do anything after waking up in the morning, or feeling pain after sitting in the same position.
Since moving slowly for a little while normally alleviates the pain, it is sometimes misunderstood as malingering or a sign of laziness.
Patients feel great pain and may even have difficulty in breathing. However, their suffering is often not understood, and feel frustrated by thoughtless remarks.
What causes palmoplantar pustulosis (PPP)?
There are important factors that have been recognized to be associated with the onset of Japanese type of PPP, and in fact, PPP often resolves with the removal of focal infections.
❐ Subtypes and terminology of palmoplantar pustulosis (PPP)
PPP was first defined in the 1930s. Two different types are recognized, and their terminology and skin manifestations have been under debate for 30 years. In 1961, the name “pustulosis palmaris et plantaris (PPP)” was adopted. At present, two subtypes of PPP are recognized, and the Japanese type, which is related to focal infections seems to be different from that encountered in Western countries, and another type which is a localized type of pustular psoriasis that is not seen in Japanese.
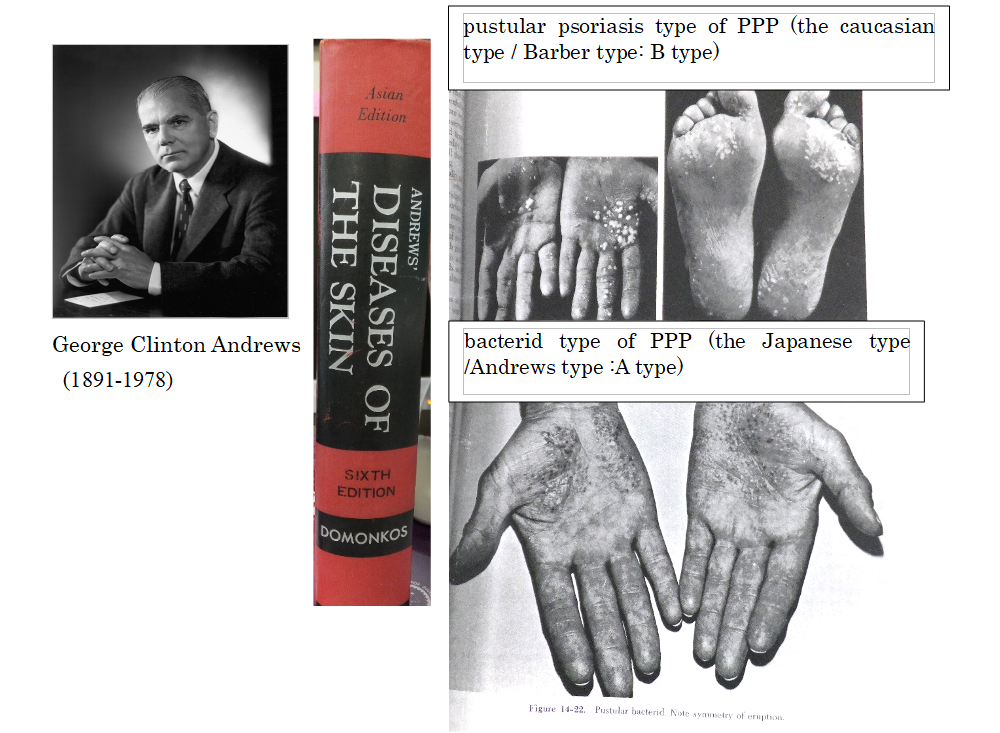
In 1930, Barber and Ingram advocated use of the term “pustular psoriasis of the extremities” to refer to psoriatic pustular reactions of the hands and feet. In 1934-1935, Andrews defined “pustular bacterid” as “pustular eruptions of the palms of the hands and soles of the feet that do not respond to topical treatment, but potentially resolve with tonsillectomy and/or dental treatment, as it is closely related to tonsillar and dental conditions”; this is the Japanese type of PPP. He thoroughly examined the two conditions, and stated in his textbook that the bacterid type of PPP (image on the bottom right) completely differs from the pustular psoriasis type of PPP (image on the upper right) in respect of the way in which the skin rashes develop. The skin rash in the pustular psoriasis type is a pustule from the beginning, while the skin rash in the bacterid type is a vesicle at first, that subsequently becomes a pustule. In other words, these two types of disease develop with completely different onset mechanisms, and the causes are also different. Focal infections of tonsil and periodontitis are only involved in the onset of bacterid type, Japanese type of PPP.
❐ What needs to be done for PPP
The prevalence rate of PPP is considered to be the highest in Japan. The national prevalence of PPP in Japan is approximately 0.12%, and the number of Japanese patients with PPP is estimated to be 135,000. These patients suffer from skin symptoms on the important functional sites of hands and feet, and severe pain associated with pustulotic arthro-osteitis (PAO), which often causes impairment of daily living activities. Therefore, efforts have been started to establish standard treatments and to develop new treatment methods by reviewing research data accumulated to date in light of progress in understanding of the immunology of the disease.
While PPP observed in western countries is localized pustular psoriasis, it is also a refractory chronic disease. Localized pustular psoriasis-type PPP can be complicated by arthritis and is called the SAPHO syndrome. Pustular psoriasis is in itself intractable, and generalized pustular psoriasis is registered as an intractable disease by the Japanese Ministry of Health, Labour and Welfare.
Development of new systemic therapies including biologics (injection and oral preparations) has advanced at a rapid pace in recent years. Considering the fact that there are 2 different types (subtypes) of PPP (the Japanese type and the caucasian type), it is important that doctors recognize and understand the other type of PPP, and assess the safety and effectiveness of each treatment while determining if each treatment is specifically effective against either type or effective against both types.
❐ Japanese type of PPP
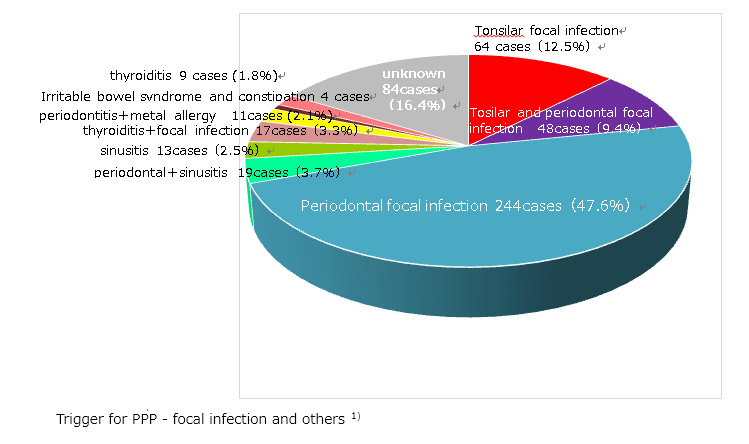
As we examine the findings of Andrews or past Japanese dermatologists, patients with PPP, it is evident that standard treatments (such as topical therapy and phototherapy) have insufficient effect in most patients. More than 80% of Japanese patients with PPP have asymptomatic focal infections in the mouth, such as periodontitis, tonsils or nasal sinuses1) , and treatment of these asymptomatic foci of infection has been observed to lead to resolution of the skin symptoms in more than 60-80% of the patients1-4)
On the other hand, PPP in Europeans and Americans resembles a type of psoriasis (pustular psoriasis in the extremities –Barber and Ingram-), which manifests pustules on the palms and soles, as well as pustules or psoriatic lesions on the arms and lower extremities. It is rare to be related with focal infections in this type of PPP.
These differences may be the reason for difficulty of understand the other side of PPP, resulted in the delay of establishing treatment strategies for them.
Another possible reason for the delay in the establishment of treatment for this condition is that the mechanism underlying its relation with “focal infection” is unknown. However, some evidences have been accumulated. PPP patients show a hyperimmune response to indigenous bacteria such as a-streptococci, due to impaired immunological tolerance towards such organisms 5) . Such a novel immune response leads to T-cell activation through the abnormal expression of secondary stimulation molecules, including cytotoxic T-lymphocyte-associated antigen 4, inducible T-cell co-stimulator and Smad7, in the tonsils of PPP patients. Activated tonsillar T cells express cutaneous lymphocyte antigen (CLA), CCR6 and β1-integrin, enter the blood circulation and are recruited to PPP skin lesions6) .
Regardless of above, no biomarkers have been established, and dermatologists, laryngologists and dentists have not yet arrived at a consensus as to how to handle asymptomatic foci of infection to date. But still, the Japanese type of PPP is expected to be cured by treatment of asymptomatic focal infections, including prevention of progression to arthro-osteitis.
1)Kobayashi S. Diagnosis and treatment for PPP. Rinsho Derma 2018; 60 (10): 1539-1544 (Japanese)
2)Clinical Characteristics of Japanese Patients with Palmoplantar Pustulosis. Yamamoto T.
3)Yamamoto Y, et al. Effects of treatment for dental focal infection on pustulosis palmaris et plantaris. Jpn J Dermatol 2001; 111: 821-826 (Japanese)
4)Murakata H, et al. Increased interleukin-6, interferon-gamma and tumor necrosis factor-alpha production by tonsillar mononuclear cells stimulated with alpha-streptococci in patients with pustulosis palmaris et plantaris. Acta Otolaryngol 1999; 119:384-91
5)Harabuchi Y, Takahara M. Pathogenic role of palatine tonsils in palmoplantar pustulosis:A review. J Detrmatol 2019; Sep doi: 10.1111/1346-8138.15100
6)Clin Drug Investig. 2019 Mar;39(3):241-252. doi: 10.1007/s40261-018-00745-6.
Trigger factors associated with the onset and aggravation of PPP
While the mechanisms of underlying the onset of PPP remain unknown, the onset of this disease is known, a history of smoking, asymptomatic tonsillitis or periodontitis (focal infection), chronic sinusitis, persistent constipation and irritable bowel syndrome are known to be triggering factors of this disease in many cases. Additionally, emotional stress often serve as a starting point for the onset.
Smoking
Smoking is highly prevalent among patients with PPP, regardless of the region of the world, and statistics indicate 60%-80% of PPP patients are smokers1) . It is unknown how smoking is related to PPP. Although PPP rarely resolves with discontinuation of smoking alone, smoking certainly seems to aggravates the symptoms4) .
In relation to its effects on the immune system, smoking is known to promote inflammation of the immune cells. It increases the release of inflammatory molecules, including interleukin-17 (IL-17), produced by the white blood cells. Smoking also aggravates periodontitis, which can induce the onset as well as aggravate PPP. Furthermore, altered nicotinic acetylcholine receptor expression has been reported to be observed strong staining to the acrosyringium in cases of PPP5) . Acrosyringium is the site of vesicle and pustule formation in PPP, and also overexpression of the nicotinic acetylcholine receptors is likely to be related to the onset of PPP. Factors possibly related to the onset of this disease have been identified little by little in recent years.
1)Akiyama T, et al. The relationships of onset and exacerbation of pustulosis palmaris et plantaris to smoking and focal infections. J Dermatol. 1995; 22: 930-934
2)Hashimoto Y, et al. A Statistical study on pustulosis palmaris et plantaris at the department of dermatology, Asahikawa Medical College during the past 17 years. Clin Dermatol 2006; 60: 633-737 (Japanese)
3)Kase K, et al. Analysis of 66 cases of pustulosis palmaris et plantaris observed at Sapporo Medical University Hospital. Jpn J Dermatol 2012; 122: 1375-1380
4)Michaëlsson G, et al. The psoriasis variant PPP can be improved after cessation of smoking. J Am Acad Dermatol. 2006 Apr;54(4):737-738
5)Hagforsen E, Edvinsson M, Nordlind K, Michaëlsson G. Expression of nicotinic receptors in the skin of patients with PPP. Br J Dermatol 2002;146: 383-91
Focal infections (such as tonsillitis, periodontitis and sinusitis)
Often, inflammation associated with an almost asymptomatic chronic infection somewhere in the body could cause another disease in a distant area of the body. This is called “focal infection.”
PPP is also a representative disease, besides IgA nephropathy, in which focal infection is known to be closely involved. Diseases which normally do not require treatment (such as asymptomatic tonsillar focal infection, asymptomatic periodontitis, chronic sinusitis, nasopharyngitis) often trigger the onset of PPP.
The causative bacteria for these focal infections are the locally commenced bacteria. In patients who are susceptible to PPP, however, thecommenced bacteria may induce an immune response and consequently lead to the onset and persistence of PPP.
Is it related to bowel symptoms, such as persistent constipation and irritable bowel syndrome?
Celiac disease, gluten sensitivity colitis, is seen in an estimated 18% of Swedish patients. Gluten sensitivity is rarely observed in Japanese patients, however, when patients with PPP are asked to report on their bowel conditions, many patients complain of persistent constipation and/or frequent diarrhea. In some cases, treatment of these bowel symptoms also leads to improvement of the skin and joint symptoms.
It has been suggested that the intestinal bacterial flora are possibly related to various chronic inflammatory diseases, including arthritis. Although it has not yet been scientifically proven as to how they may be related, the intestinal immune system has been reported to be involved in the development of ulcerative colitis and Crohn’s disease. IL-17 is known as an important factor, but he precise role of IL-17 has not yet been identified especially in inflammatory bowel diseases.
Much of the details of the intestinal immune system remain unknown. Taking excessive doses of certain intestinal bacteria at one’s own judgment is not only not helpful, but also dangerous.
Allergy to dental metal
A causal relationship has been reported between onset of PPP and dental metal allergy. On the other hand, we have encountered many patients with PPP in whom the eruptions did not resolve with removal of the dental metals alone. In many cases, both removal of dental metals and treatment of dental focal infection are performed simultaneously, and it still remains under debate as to which of the two could be responsible for improving the symptoms of PPP.
When the patients who were treated for dental focal infections and those who underwent dental metal removal were strictly differentiated, PPP seemed to be related to dental metal allergy in only about 5% of cases1) . A cohort study conducted recently showed that most patients who had undergone dental metal removal had also undergone treatments for focal dental infections at the same time, indicating that dental metal allergy may not, after all, be the main cause of PPP. Therefore, easy removal of dental metals is not recommended2) .
1)Kobayashi S. Diagnosis and treatment for PPP. Rinsho Derma 2018; 60 (10): 1539-1544 (Japanese)
2)Masui Y, et al. Dental metal allergy is not the main cause of PPP. J Eur Acad Dermatol Venereol. 2019 Jan 17. doi: 10.1111/jdv.15434
Comorbidities of palmoplantar pustulosis
Palmoplantar pustulosis (PPP) is not a disease involving the skin alone.
The clinical manifestations of PPP could also include bone and joint inflammation, besides skin symptoms (refer to Joint and bone symptoms – Pustulotic arthro-osteitis (PAO) in SAPHO syndrome associated with PPP1) . Also, asymptomatic tonsillar lesions, sinusitis and odontogenic infections are well known to predispose to the onset of PPP (refer to What causes palmoplantar pustulosis (PPP)?).
Additionally, close attention must be paid to possibly underlying diseases such as diabetes mellitus, autoimmune thyroiditis or hyperlipidemia (increased cholesterol and triglyceride levels), as well as to bowel symptoms such as constipation and diarrhea.
❐ If you have diabetes mellitus and/or autoimmune thyroiditis, you should visit a specialist in order to keep these conditions under good control.
Autoimmune thyroiditis is an autoimmune diseases in which autoantibodies attack one’s own thyroid tissues; Basedow’s disease (thyroid hyperfunction disorder) and Hashimoto’s disease (thyroid hypofunction disorder). When PPP patients have autoimmune thyroiditis and/or poorly controlled diabetes mellitus, the responses for the treatments often tend to be poor even they are adequate. It is also not rare that PPP becomes a clue to recognize the existence of autoimmune thyroiditis and/or diabetes mellitus using the blood test.
The prevalence rate of diabetes mellitus in Japanese patients with PPP is reported about 10%1,2) , which is not particularly high. In Swedish women, the prevalence rate of diabetes mellitus in PPP patients is reported as high as 28% 3) .
It is still unclear how diabetes mellitus is related to aggravation of PPP. A close relationship is known to exist between smoking and diabetes mellitus, diabetes mellitus and periodontitis, and smoking and periodontitis. It is also considered that these conditions aggravate one another. In severe cases of periodontitis, periodontal bacteria constantly enter the bloodstream and activate macrophages. Activated macrophages accumulate in adipose tissue and stimulate to produce inflammatory cytokines (adipokines) such as tumor necrosis factor-alpha (TNFa) and interleukin-6 (IL-6), leading to increase of insulin resistance and the risk of diabetes mellitus4) .
The reported prevalence rate of underlying autoimmune thyroiditis (including Basedow’s disease and Hashimoto’s disease) in patients with PPP is 12%3) in Swedish women and 4.4~5.1% 1,5) in Japanese, which is not so high. Skin symptoms of PPP may become aggravated with aggravation of the thyroiditis. In addition, PPP patients with autoimmune thyroiditis may not completely resolve even after removal of focal infections. Further investigation is need to determine why the patients with autoimmune thyroiditis have difficulty to cure PPP. The mucosa-associated lymphoid tissue (MALT) is one of the sites of microbes entry and consists with gastrointestinal tract, oral passage including tonsil, nasopharyngeal tract, thyroid, salivary gland, and skin. MALT may be related to tonsillar focal infectious diseases6) .
❐ Dislipidemia is frequently observed in PPP
Dislipidemia (high level serum cholesterol and/or triglyceride) is frequently observed in patients with PPP 2) . Unlike in psoriasis, the body mass index (BMI) is not particularly high in PPP patients. Although the mechanism remains unknown, it may be related to chronic inflammation. As for hypertension, there are conflicting data. While one study has reported a high incidence of hypertension in patients with PPP, other showed much lower rate. The lower rate is 11.9%2) , and higher rate is 25%1) . This is another issue that needs to be resolved in the future.
Furthermore, emotional stress is considered to greatly affect onset and aggravation of palmoplantar pustulosis. Psychologic disorders such as depression is assumed to be one of factors which prevent improvement of palmoplantar pustulosis. You need to consult with physicians specialized in psychosomatic medicines and psychiatry if necessary.
❐ Psychological disorder
Psychologic disorders, such as depression, are observed in some PPP patients. It is reported that about 4% of PPP patients has psychological disorder 1) .
❐ Constipation, irritable bowel syndrome, and intestinal regulation
The relationship between imbalance of intestinal bacteria (dysbiosis) and PPP has been attracting attention. Some PPP patients have persistent constipation, or repeated episodes of bowel symptoms such as diarrhea and mild constipation5) . Since studies on the relationship between intestinal bacteria and immunity have just begun, there is not sufficient medical expertise in the field. The surface area of the intestine is about 1.5 times as large as that of a tennis court, and various intestinal bacteria reside in such a large area.
The intestinal environment began to be considered as an important factor in patients with PPP, when it was reported that incidence rate of gluten sensitivity or other enterocolitis was high among Swedish patients with PPP3) .
Clinical manifestations of inflammatory bowel diseases, such as Crohn’s disease, indicate the existence of a relationship between intestinal bacteria and arthritis. Furthermore, injection of intestinal bacteria isolated from a patient with rheumatoid arthritis into a mouse model has been shown to cause arthritis in the mouse. This also suggests the existence of a relationship between intestinal bacteria and arthritis7,8) .
Oral butyric acid can improve colonocyte regulation and bacterial microflora composition by exerting anti-inflammatory activity in intestine, and in some occasion helped to improve the skin eruptions in some patients with PPP. However, butyric acid is not effective in all patients with PPP. In fact, some patients develop diarrhea and/or abdominal distension after taking butyric acid. Thus, there are differences among individual PPP patients in the significance of the intestinal bacterial microflora. It is considered to be ideal for the intestinal bacterial microbiota to comprise a wide variety. It is well known that intestinal flora is called “intestinal microflora,” by reference to the phrase “garden of flowers.” It is necessary to study the relationship between PPP and bowel symptoms/characteristics of the intestinal microbiota in Japanese patients as well.
1)Hiraiwa T, Yamamoto T: Comorbidities of Japanese patients with palmoplantar pustulosis: a report from a single centaer, Int J Dermatol 2018; 57: e40-e41.
2)Miyata R, et al. Comorbidities of Japanese patients with palmoplantar pustulosis. The 116th Annual Meeting of the Japanese Dermatological Association (Sendai) 2017; P-324.
3)Hagforsen E, et al: Women with PPP have disturbed calcium homeostasis and a high prevalence of diabetes mellitus and psychiatric disorders: a case-control study. Acta Derm Venereol 2005; 85: 225-232.
4)Nishimura F, et al: Periodontal disease and diabetes mellitus: the role of tumor necrosis factor-alpha in a 2-way relationship. J Periodontol 2003; 74: 97-102.
5)Kobayashi S: Diagnosis and Treatment for PPP. Hifu Rinsho (Japanese) 2018; 60: 1539-1544.
6)Harabuchi Y, Takahara M. Recent advances in the immunological understanding of association between tonsil and immunoglobulin A nephropathy as a tonsil‐induced autoimmune/inflammatory syndrome. Immun Inflamm Dis. 2019; 7: 86-93
7)Brusca AB, et al: Microbiome and mucosal inflammation as extra-articular triggers for rheumatoid arthritis and autoimmunity. Curr Opin Rheumatol 2014; 26: 101-107.
8)Maeda Y, et al: Dysbiosis contributes to arthritis development via activation of autoreactive T cells in the intestine. Arthritis & Rheumatol 2016; 68: 2646-2661.
Is it true that Japanese patients are more susceptible to palmoplantar pustulosis (PPP)?
Yes, it is true. The prevalence is high in the Japanese and Swedish, and relatively high in Chinese and Koreans.
The prevalence of a disease may vary among countries. One of the speculated reasons is the differences in the human leukocyte antigen (HLA) profile.
HLA determines whether or not rejection would occur at the time of organ transplantation, and decides how the immune system responds to each foreign object, such as bacteria or viruses. The HLA system is a gene complex that is responsible for the diversity of immune responses.
The susceptible HLA type for PPP has not yet been identified. About 5-6% of patients with PPP have a family member (parent, sibling or relative) with the same disease. However, HLA is like immunological predisposition passed on from generation to generation, and there is an extremely large number of HLA patterns. It is unnecessary to worry about passing PPP to children.
Identification of the susceptible HLA type for PPP may help in determining why such immune responses occur, why focal infection is a strong trigger factor in Japanese patients with PPP, and how to treat the condition in the future.
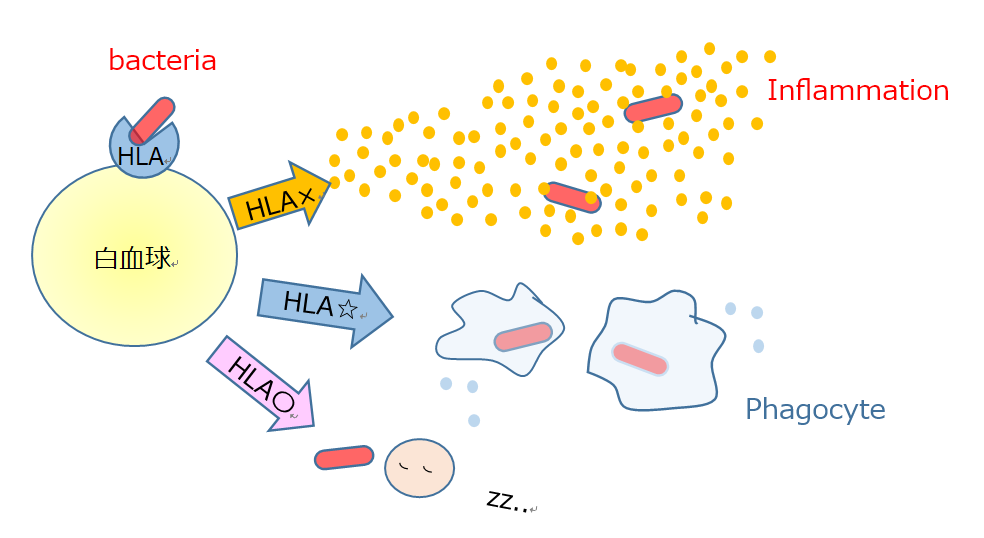
HLA and diversity of immune response
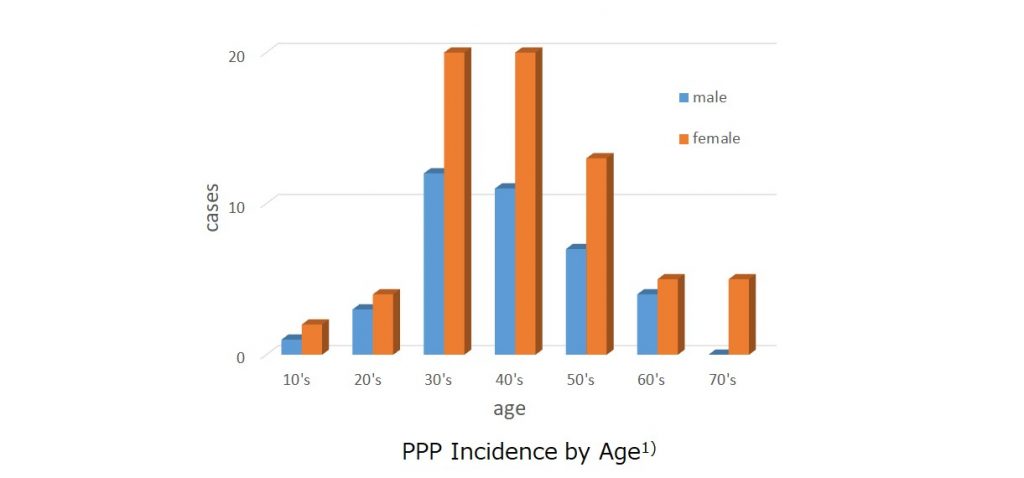
❐ The male: female ratio of PPP is about 1:2, and women in their 30s to 50s are more susceptible to PPP.
Statistics from various institutions in Japan have indicated a predisposition of middle- aged women for this disease.
In a study of PPP conducted in the year 2010 in response to a request from national health insurance societies throughout Japan, the male:female ratio was found to be around 1:2, and the incidence rate was the highest among patients in their 30s to 50s.
The number of patients with PPP in Japan is estimated to be 135,000, and the calculated prevalence rate is 0.12%.
The disease is rarely encountered in children.
1. Fujishiro K, et al. The Japanese Journal of Dermatology, 2015; 125: 1775-1782
2. Kubota K, et al. Epidemiology of psoriasis and PPP: a nationwide study using the Japanese national claims database. BMJ Open, 2015: 5: e006450. Doi: 10.1136/bmjopen-2014-006450
3. Akiyama T, et al. The relationships of onset and exacerbation of pustulosis palmaris et plantaris to smoking and focal infections. J Dermatol. 1995; 22: 930-934
Which symptom is observed first, the skin pustules or the bone/joint pain?
These symptoms often start around the same time.
It is important to recognize that some patients with PPP can present with bone and joint pain.
❐ Pustules and bone pain occur simultaneously in most cases
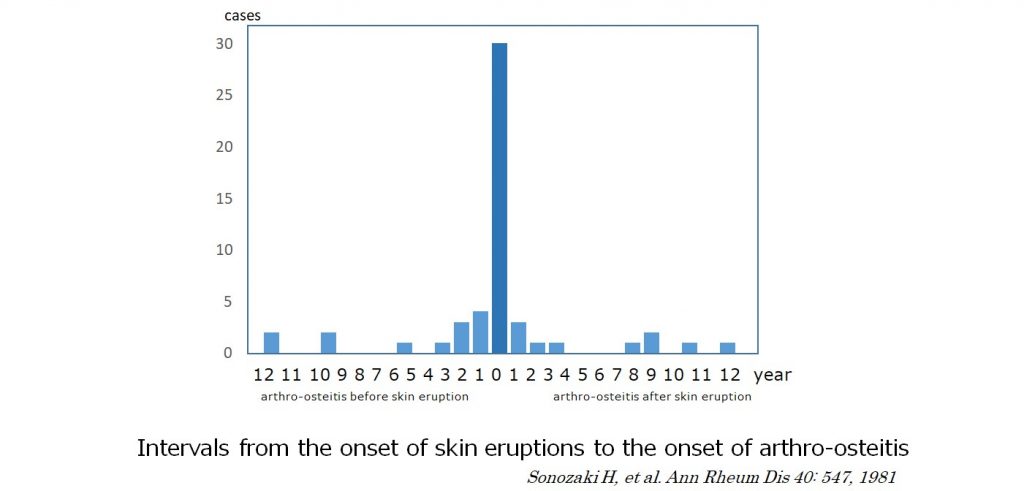
Sonozaki H, et al. reported that pustulotic arthro-osteitis (PAO) is most often observed in the anterior chest wall (sternoclavicular arthritis of the clavicle, sternum and ribs), shoulders, spine and various other parts of the body, and that more than 70% of the patients develop pustulosis and PAO simultaneously within 2 years difference. On the contrary, 80% of psoriasis patients develop skin lesions before psoriatic arthritis. Furthermore, there were cases that showed pustulosis many years after the occurrence of arthro-osteitis. In patients with pustules on the palms and soles, chest or back pain may be suspected to be caused by pustulotic arthro-osteitis. On the other hand, in patients presenting with back pain alone, it could take many years before an accurate diagnosis is made.
It is important to suspect the possibility of PAO in patients with pain in the chest wall or back without any obvious cause. If it is “pain at rest” (the patient experiences the most severe pain when waking up in the morning or after prolonged rest), the attending physician must be notified.
In PAO, the pain may resolve and recur repeatedly. To confirm the diagnosis, radiological examination is necessary.
Is it curable?
PPP is a curable disease. However, while the skin symptoms would disappear no matter how long a person has suffered from PPP, the bone symptoms may not completely resolve unless they are treated in the early phase.
❐ Skin symptoms of PPP are curable.
In most cases, the skin symptoms are curable. A trigger for onset of the disease can be identified in most Japanese patients with PPP. If the trigger factors are discovered and removed, the skin symptoms also usually resolve within 1-2 years, or at least improve to become merely rough skin.
Various medical institutions have published their statistics on the average time to resolution of PPP. The average reported time to resolution varies from 5 years and 7 years to 13 years. The wide range reports show that PPP does not easy resolve unless physicians focus on removing the trigger factors.
Asymptomatic inflammation of the tonsils, periodontal tissues and nasal sinuses (called focal infection*) are recognized to be involved in the onset of PPP in most Japanese patients (refer to What causes palmoplantar pustulosis (PPP)?).
There are 3 important points you should pay attention to. First, foci are usually asymptomatic. Since such inflammation is rarely accompanied by noticeable symptoms such as pain or swelling, it is normally overlooked and untreated. In this sense, if such treatments as tonsillectomy or dental treatments are sometimes considered as overtreatment by some physicians. Secondly, it is not infection but excessive immune reactions against the commensal bacteria. So it takes 1 or 2 years even after removal of foci. Thirdly, in the middle of the treatment of foci, the symptoms may get worse. Unless you are aware of those facts, you may feel uneasy or you may misunderstand that you may not be receiving the right treatments.
PPP causes continuous pustulation and peeling of skin, and becomes a psychological and physical burden on the patients, preventing them from normally carrying out their normal daily life activities. To achieve resolution of the skin eruptions, therefore, it is important to reflect on the previous clinical course of the patient and to search for trigger factors. It would be advisable to visit a dentist and undergo dental examinations and dental x-rays to detect the presence/absence of root infection/pyorrhea, and inform your dermatologist of the findings. Bowel symptoms (diarrhea and constipation), and although uncommon, metal allergy (to dental filling metals) are also known trigger factors. Identification and removal of the trigger factors often lead to improvement of the symptoms of PPP. Smoking cessation does not normally lead to resolution of PPP. However, it is still important to quit smoking, because the cause of PPP may be closely related to cigarette smoking.
In some cases, no trigger factors are found. In other cases, removal of the trigger factors fails to lead to complete resolution of the lesions. Such patients could choose to receive other treatments to suppress their overactive immune responses (such as phototherapy, oral therapy or injection of biologics). If you are such a patient, think positively and stay actively engaged in your treatment while consulting your attending physician.
* When you have chronic inflammation somewhere in your body, this chronic inflammation can trigger a lesion (PPP in this case) in other parts of your body. Such chronic inflammation is called “focal infection.” One such example is tonsillar infection and IgA nephropathy.
Please refer to “Treatment for PPP” for details of each therapy.
❐ Complete resolution of bone inflammation may be difficult to achieve.
Sonozaki et al. 1) stated that pustulotic arthro-osteitis (PAO) rarely leads to functional disorders (as seen, for example, in cases of rheumatoid arthritis) and has a relatively good prognosis. However, even patients with mild bone/joint symptoms at the beginning could eventually develop serious functional disorders in some cases. Most patients have sternoclavicular joint arthritis at first, and then after a few to about ten years, spondyloarthritis may occur. The inflammation causes bone proliferation, and in the case of involvement of the spine, the upper and lower vertebrae fuse to result in a stiff unbendable spine (bamboo spine) and spinal compression fractures. It was reported in a cohort study of Chinese patients with bone and joint inflammation (including 143 patients with PAO), that discontinuation of treatment led to exacerbation of bone and joint inflammation2). In cases with trigger factors for PPP, such as focal infection, the bone inflammation may aggravate while the skin symptoms resolve, unless the trigger factor is removed.
Since skin is an organ with regenerative ability, it recovers to normal. On the other hand, destruction and deformity of bones results in sequelae. Like the skin eruptions, bone/joint symptoms of PAO are often associated with a focal infection of the tonsils and/or the teeth. Therefore, leaving a focal infection untreated may lead to progression of the bone/joint symptoms in cases of PPP.
1)Sonozaki H, et al. Clinical features of 53 cases with pustulotic arthro-osteitis. Ann Rheum Dis 1981; 40: 547-553
2)Li C, et al. Synovitis, acne, pustulosis, hyperostosis and osteitis syndrome: a single center study of a cohort of 164 patients. 2016; 55: 1023-1030
Which clinical department should I visit?
First of all, it is important that you are correctly diagnosed with PPP. Therefore, we recommend that you consult with dermatologist at first.
A dermatologist should be able to make a diagnosis of PPP. Since several other diseases can mimic this condition (including pompholyx, dyshidrotic eczema, tinea (athlete’s foot and tinea of hand), psoriasis of the hands and feet), differential diagnosis by a dermatologist is necessary.
Furthermore, some patients may develop inflammation of the joints and bones, and complain of swelling and pain (pustulotic arthro-osteitis (PAO)).Joint and bone symptoms – Pustulotic arthro-osteitis (PAO) in SAPHO syndrome, associated with palmoplantar pustulosis (PPP) PAO often involves the sternoclavicular joints (joints around the base of the neck on the front of the chest wall), spine and the buttock region (including spondylitis, sacroiliac arthritis, hip joints), as well as the joints and bones of the hands and feet. It is necessary for dermatologists to coordinate with orthopedists and rheumatologists in the management of these patients. In addition, orthopedists and rheumatologists also need information about the skin symptoms and should determine which symptoms should be treated first. If any swelling and/or pain in the joints is noted, the attending dermatologist must be notified.
Focal infections can trigger the onset of PPP. You should receive treatment at the otorhinolaryngology department if a focal infection is diagnosed in the tonsils or nasal sinuses, and in the dental or periodontal treatments if a focal infection is diagnosed at the base of a tooth root or the surrounding teeth. These conditions usually do not require treatment. Therefore, it is necessary for a dermatologist to specifically request for treatment of these focal infections at these clinical departments in order to cure PPP or PAO.
Satomi Kobayashi

